Types of Chemical Reactions
Types of Chemical Reactions: Overview
This topic covers concepts, such as Types of Chemical Reactions, Combination Reactions, Reaction between Calcium Oxide and Water, Reaction between Calcium Hydroxide and Carbon Dioxide, Applications of Calcium Hydroxide, Burning of Coal, Formation of Water, Exothermic Reactions, Burning of Natural Gas, Respiration as an Exothermic Reaction, Decomposition of Vegetable Matter into Compost, Decomposition Reactions, Thermal Decomposition Reactions, Decomposition of Ferrous Sulphate, Decomposition of Limestone, Decomposition of Lead Nitrate, Electrolysis of Water, Photochemical Decomposition Reactions, Decomposition of Silver Chloride, Decomposition of Silver Bromide, Endothermic Reactions, Displacement Reactions, Reaction between Iron and Copper Sulphate, Reaction between Zinc and Copper Sulphate, Reaction between Lead and Copper Chloride, Double Displacement Reactions, Precipitation Reactions, Reaction of Sodium Sulphate with Barium Chloride, Redox Reactions, Oxidation Reactions, Reduction Reactions, Oxidising Agents, Reducing Agents, Reaction between Copper and Oxygen, Reaction between Copper Oxide and Hydrogen, Reaction between Zinc Oxide and Carbon & Reaction between Hydrochloric Acid and Manganese Dioxide etc.
Important Questions on Types of Chemical Reactions
For welding a mixture of oxygen and_____ is burnt.
When lead nitrate is heated a brown gas is evolved, the evolved gas is
Which fuel has the highest calorific value?
Choose the correct statements about the given chemical reaction.
a. Iron is getting oxidised.
b. Water is getting reduced.
c. Water is acting as a reducing agent.
d. Water is acting as an oxidising agent
An example (s) for endothermic process (es) is (are)
a. Dilution of sulphuric acid
b. Sublimation of dry ice
c. Condensation of water vapours
d. Evaporation of water
The type of the above reaction is:
The metal oxide which decomposes on heating
Which of the following arrangements represent the increasing oxidation number of the central atom ?
Which one is addition reaction?
The oxidation reaction in the following chemical changes is
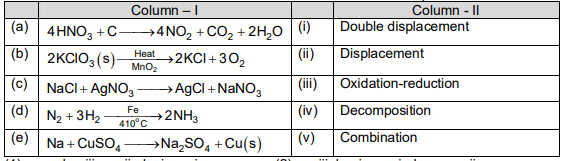
Match the items of the column -I with column-II and choose the correct option.
A substance that oxidises itself and reduces other is-
The above reaction is a-
In which reaction the addition and removal of oxygen take place simultaneously?
Which of the following are combination reaction?
(i)
(ii)
(iii)
(iv)
In the reaction the oxidising agent is:
In the equation , the substance reduced is
What happens when dilute hydrochloric acid is added to iron filings?
Excess of reacts with solution and then solution is added to it. Which of the following statements is incorrect?
Which of the following reaction is not a redox reaction ?
